| Element | Electron Configuration | Electronegativity |
| Potassium | 4s1 | 0.82 |
| Phosphorus | 3p3 | 2.19 |
| Oxygen | 2p4 | 3.44 |
| Element | Bohr-Rutherford Diagram | Lewis Dot Diagram |
| Potassium |  |
 |
| Phosphorus | 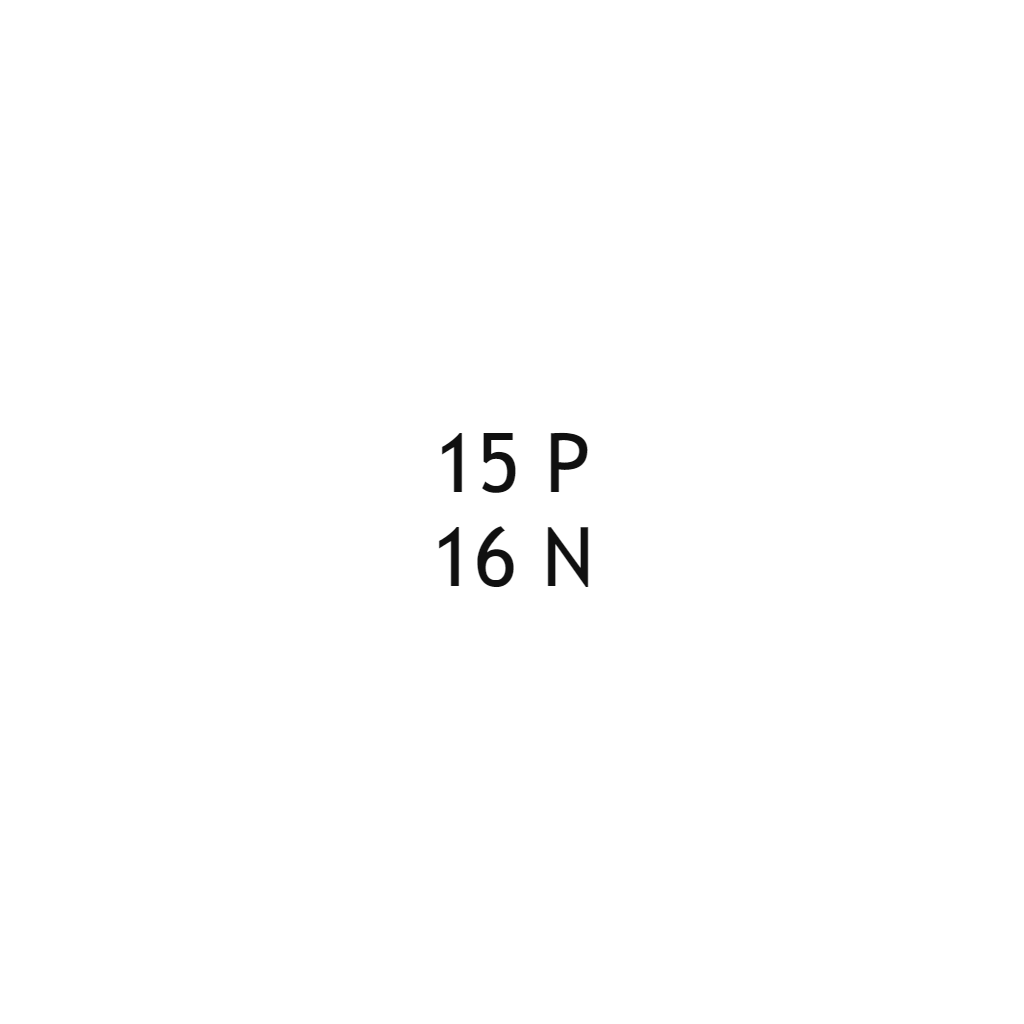 |
 |
| Oxygen | 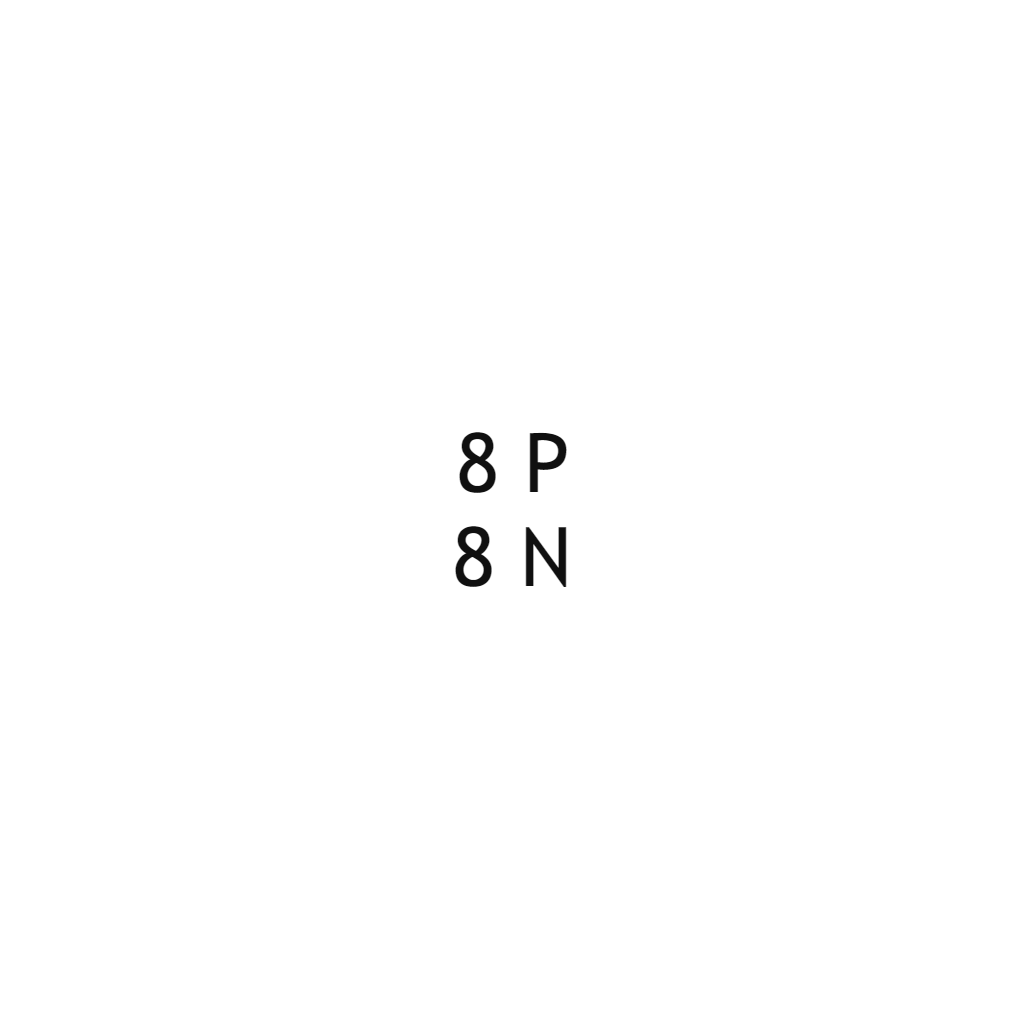 |
 |
| Compound Diagrams | ||
| Phosphate |  |
|
| Potassium Phosphate |  |
Flame Test for Bonding Metal
Potassium burns with a light purple (lilac) colour.
The Compound
| Chemical Formula | K₃PO₄ |
| Classical | Potassium Phosphate |
| IUPAC | Tripotassium Phosphate |
| Chemical Formula | K₃PO₄ • H₂O |
| Classical | Potassium Phosphate Monohydrate |
| IUPAC | Tripotassium Phosphate Monohydrate |
Chemical Reactions
| Example of a Decomposition Reaction | K₃PO₄(s) + H₂O(L) → 3K(aq) + PO₄(aq) |
| Example of a Single Displacement Reaction | K₃PO₄(aq) + Au(s) → AuPO₄(s) + 3K(aq) |
| Example of a Double Displacement Reaction | K₃PO₄(aq) + 3AgNO₃(aq) → Ag₃PO₄(s) + 3KNO₃(aq) |
Potassium Phosphate's solubility in water is 50.8g/100mL
The Mole
| Potassium Molar Mass | 39.10u | Phosphate Molar Mass | 30.97u + 4(16.00u) = 94.97u | |
| Phosphorus Molar Mass | 30.97u | Potassium Phosphate Molar Mass | 3(39.10u) + 94.97u = 212.27u | |
| Oxygen Molar Mass | 16.00u |
Example of a Calculation using Avogadro's Number
212.27u x 6.022x10^23 = 1.27x10^26u is the mass of one mole of Potassium Phosphate.
Example of a Calculation using Mass
n x M = m
4 x 1.27x10^26u = 5.08x10^26g
There are 5.08x10^26g in 4 moles of K₃PO₄
Example of a Calculation of Percent Composition
100 ÷ 212.27u = 0.47
3(39.10u) x 0.47 = 55.131% Potassium
30.97u x 0.47 = 14.5559% Phosphorus
4(16.00u) x 0.47 = 30.08% Oxygen
55.131 + 14.5559 + 30.08 = 99.7669 = ~100% Potassium Phosphate
Empirical Formula
K₃PO₄
The empirical formula for potassium phosphate is exactly the same as the chemical formula.
Stoichiometry
Molar ratios in an equation:
2 moles of potassium phosphate react with 3 moles of gold.
K₃PO₄(aq) + Au(s) → AuPO₄(s) + 3K(aq)
1 : 1 : 1 : 3
2/1 3/1
2<3
K₃PO₄ is the limiting reagent.
Au is the excess reagent.
Properties of Solutions/Acids and Bases
Dissociation of K₃PO₄ in water:
K₃PO₄(s) + H₂O(L) → 3K⁺(aq) + PO₄⁻³(aq) + H₂O(L)
Example of K₃PO₄ being a product of a neutralization reaction:
H₃PO₄(aq) + KOH(aq) → K₃PO₄(aq) + H₂O(L)
Solubility and Reactions
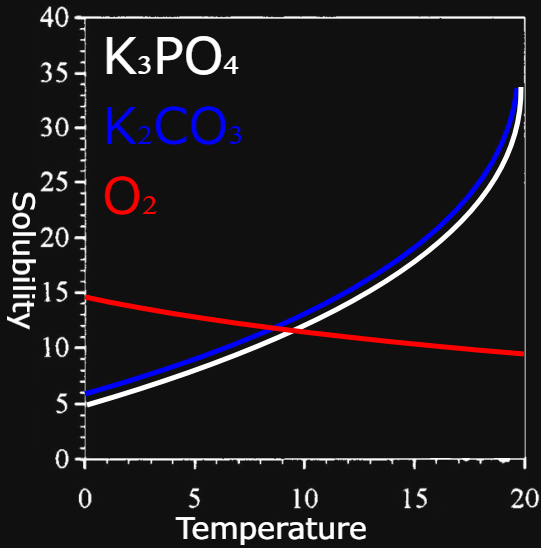
The solubility curve of K₃PO₄ compared to K₂CO₃ and O₂: